A cement is a binder. It is a substance that hardens and adheres to other materials to bind them together. Cement is one of the most important man-made substances in the world today. In this article, we will look at the cement manufacturing process, its applications, some types of cement and cement testing.
Applications of the Different Types of Cement
Cement can be used alone as grouting material, but its most common application is in mortar and concrete, where it is combined with an inert substance called an aggregate. Mortar refers to cement combined with sand or crushed stones that is no larger than 5 mm in size. It finds uses in holding bricks, blocks, and stone together in buildings or as surface finish renderings.
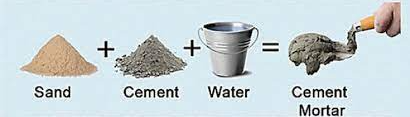
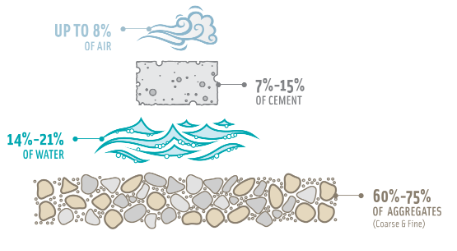
Concrete is a combination of cement, sand or other fine aggregates, and a coarse aggregate ranging in size from 19 to 25 mm. People use concrete in a wide range of construction applications.
Some applications of the different types of cement include:

- Plastering, masonry construction, pointing, and other purposes.
- Making drain and pipe joints.
- Ensuring that construction is watertight.
- Laying floors, roofs, beams, staircases, and pillars, among other things.
- When a hard surface is necessary to protect exposed surfaces of structures from weather-related damage and certain organic or inorganic pollutants.
- Production of precast pipes, piles, and fence posts, among other things.
- Building of significant engineering constructions such as bridges, culverts, dams, tunnels, and lighthouses, among others.
- Construction of foundations, waterproof flooring, and pathways, among other things.
- Building wells, water tanks, lamp posts, tennis courts, telephone cabins, roads etc.
Raw Ingredients Required for Cement

Cement primarily consists of lime (calcium oxide, CaO), silica (silicon dioxide, SiO2) and alumina (aluminium oxide, Al2O3). The lime comes from a calcareous raw material, whereas the other oxides come from an argillaceous or clayey source. Manufacturers add small amounts of other raw materials, such as clay, iron oxide (Fe2O3), fly ash, Calcium Carbonate (CaCO3), and bauxite to achieve the necessary composition. Different types of cement have different quantities of these ingredients. Lastly, manufacturers add gypsum to the burnt cement clinker during grinding to manage the cement’s setting time.
Cement Manufacturing Process
Cement Manufacturing is a four-step process, that is as follows:
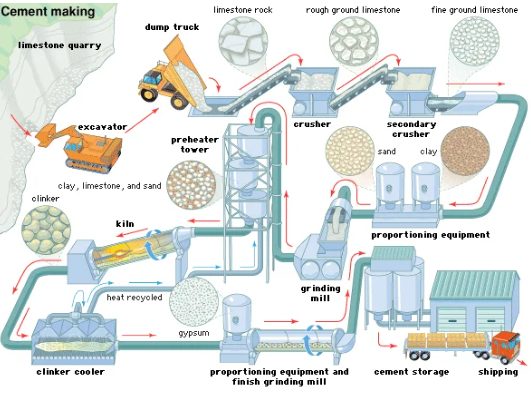
Grinding and crushing
Large revolving, cylindrical ball mills, or tube mills carrying steel grinding balls crush all materials except for soft materials, in two stages. Depending on the procedure, this grinding can be done wet or dry, although, for dry grinding, raw materials may need to be dried in cylindrical, rotating dryers beforehand.
In wash mills, soft materials break down because of vigorous swirling with water, resulting in a fine slurry that passes through screens to eliminate large particles.
Blending
In large silos, workers mix raw materials in appropriate quantities to obtain a preliminary approximation of the chemical composition necessary for a specific cement. Agitation and rapid circulation provided by compressed air ensure thorough mixing of the materials in large silos. The wet raw material slurry, which includes 35 to 45 per cent water, is sometimes filtered to reduce the water content to 20 to 30 per cent. This filter cake is then fed into the kiln.
Burning
Bottle kilns were the first kilns used in the manufacturing process, where cement was burnt in batches. Following this, chamber kilns and subsequently continuous shaft kilns became popular. These kilns may be up to 200 metres long and six metres in diameter in wet process plants and much shorter in dry process plants. They consist of a steel, cylindrical shell, coated with refractory materials. Furthermore, they revolve slowly on a slightly inclined axis. The raw material feed enters from the higher end of the kiln and flows gently down to the lower, or fire, end.
The fuel used for burning includes pulverized coal, natural gas, or oil pumped through a pipe. Depending on the raw materials burnt, the temperature at the firing end ranges from roughly 1,350 °C to 1,550 °C. At the rear end of the kiln, a heat exchanger maximises heat transmission through the entering raw materials and thus, minimises heat loss from the waste gases. The burnt product exits from the kiln as little clinker nodules. These clinkers pass through many coolers, where heat gets transferred to the incoming air, cooling down the product. Manufactures process clinker into cement immediately or keep it in stockpiles for later use.
Grinding
Horizontal mills, similar to those used to grind raw materials, grind the clinker and the needed amount of gypsum to a fine powder. Workers may feed the material directly into the mill, and then, separate the coarser material from the ground product and feed it back into the mill for additional processing.
Chemical Reactions During the Cement Manufacturing Process
Here are the different chemical reactions that occur during the cement manufacturing process.
Step 1:
During the calcination reaction, limestone is burnt to remove the carbon, producing lime (CaO). This step is the largest global CO2 emitter.
CaCO3 -> CaO + CO2
Step 2:
The lime reacts with silicon dioxide (SiO2) to produce dicalcium silicate (2CaO.SiO2) and tricalcium silicate (3CaO.SiO2)
2CaO + SiO2 -> 2CaO.SiO2
3CaO + SiO2 -> 3CaO.SiO2
The lime also reacts with aluminium oxide (Al2O3) to form tricalcium aluminate (3CaO.Al2O3)
3CaO + Al2O3 -> 3CaO.Al2O3
Step 3:
In the last step, calcium oxide, aluminium oxide, and ferric oxide react together to form cement (4CaO.Al2O3.Fe2O3)
4CaO + Al2O3 + Fe2O3 -> 4CaO.Al2O3.Fe2O3
Workers pump the finished cement into storage silos, from where it goes for packaging in paper bags or shipment in bulk containers.
The 13 Types of Cement
There are different types of cement for different construction works. Here is a list of the 13 types of cement.

Ordinary Portland Cement (OPC)
It is the most widely manufactured and utilised form of cement in the world, with an annual worldwide production of over 3.8 million cubic metres. This cement is appropriate for all types of concrete construction.
Portland Pozzolana Cement (PPC)
Grinding pozzolanic clinker with Ordinary Portland cement creates Portland pozzolana cement. Combining pozzolana with gypsum or calcium sulphate, or intimately and evenly mixing Portland cement and fine pozzolana can also make this type of cement. When compared to standard portland cement, this cement has great resistance to different chemical attacks and therefore people commonly use it in concrete. PPC also finds use in sewage works, maritime constructions, as well as underwater construction.
Quick Hardening Cement
This type of cement achieves strength quickly. It has a greater lime concentration, higher Ca3SiO content, and is finer, resulting in stronger strength development than OPC. The strength of quick hardening cement after three days is comparable to the strength of OPC after seven days with the same water-cement ratio. As a result, the benefit of this cement is that construction workers may remove the formwork sooner, increasing the tempo and lowering the cost of construction. Prefabricated concrete buildings, road construction etc, use quick hardening cement.
Quick-Drying Cement
The difference between quick-drying cement and quick hardening cement is that quick-drying cement sets sooner, while rapid hardening cement builds strength quickly. Workers can remove formwork early in both circumstances. Concreting in static or moving water and in rainy or cold weather necessitates the use of quick-setting cement.
Low Heat Cement
Manufacturers make this cement by increasing the quantity of Ca2Si04 while keeping the percentage of tricalcium aluminate below 6%. The addition of a tiny amount of tricalcium aluminate causes the concrete to have a low heat of hydration. Low heat cement is excellent for mass concrete construction such as gravity dams because the low heat of hydration reduces concrete breaking due to heat. Furthermore, this cement has stronger sulphate resistance, is less reactive, and has a shorter initial setting time than OPC.
Sulphate Resistant Cement
This type of cement limits the danger of sulphate attack on concrete and thus, people use it to build foundations in areas with high sulphate content. The amount of tricalcium aluminate and Tetracalcium aluminoferrite in this cement is less compared to others. Sulfate-resistant cement is used in construction that is subjected to harsh sulphate action by water and soil, such as culverts, canal linings, retaining walls, syphons, and so on.
Blast Furnace Slag Cement
Blast furnace slag cement is made by grinding clinkers with around 60% slag and has characteristics similar to Portland cement. People generally use it for projects with a lower cost of construction.
High Alumina Cement
Manufacturers make high alumina cement by melting a bauxite-lime combination and mixing it with clinker. This quick-setting cement has an initial and final setting time of 3.5 and 5 hours, respectively. This cement has higher compressive strength and is more workable than standard Portland cement. Therefore, people use this type of cement for applications where concrete is exposed to high temperatures, frost, and acidic action.
White Cement
This white variety of conventional Portland cement consists of the basic ingredients but no iron oxide. It is more expensive. People use this type of cement for architectural purposes such as in facing panels, precast curtain walls, terrazzo surfaces, as well as for interior and exterior decorative work. Some examples of decorative work include facing slabs, floorings, ornamental concrete products, swimming pools, garden paths, and so on.
Coloured Cement
Manufacturers make this cement by combining 5- 10% mineral colours with conventional cement. It is commonly used for floor decoration.
Air entraining Cement
Manufacturers make this cement by grinding clinker with indigenous air-entraining substances such as glues, resins, sodium salts of sulphates, and so on. This type of cement is particularly well suited for improving concrete workability with a lower water-cement ratio and frost resistance.
Expansive Cement
This type of cement expands over time and does not contract during or after hardening. People primarily use the cement in the grouting of anchor bolts and prestressed concrete ducts.
Hydrophobic Cement
MAnufacturers produce hydrophobic cement by combining water-repelling compounds. It has great workability and strength. Furthermore, it repels water and remains unaffected throughout the monsoon or showers. Water structures such as dams, water tanks and spillways, utilise hydrophobic cement.
Cement Testing
National cement requirements provide many tests in order to govern the fineness, setting time, soundness, and strength of the cement. These tests are as follows:
Fineness

Sieve tests were once employed to regulate fineness, but more sophisticated procedures are now widely utilised. The most typical approach, used for both grinding process control and completed cement testing, determines the surface area per unit weight of the cement. To do so, workers calculate the rate of airflow through a bed of cement. Other methods rely on the rate of sedimentation of the cement in kerosene or elutriation (separation) in an airstream to determine particle size distribution.
Soundness
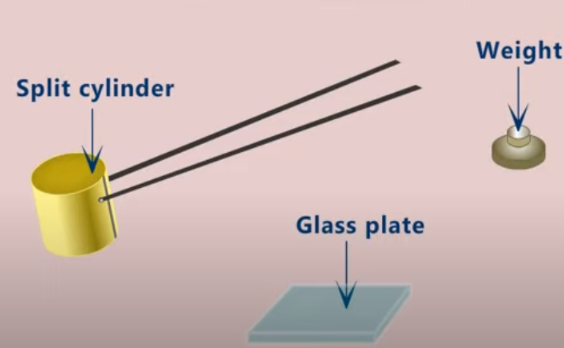
A cement must not expand significantly after it has set, since this might cause a mortar or concrete to crack. This soundness quality is verified by exposing the set cement to boiling water or high-pressure steam. Unsoundness can result from the presence of too much free magnesia or hard-burned free lime in the cement.
Setting Time

Setting and hardening of cement is a continuous process, but, the two stages are separated for testing reasons. The first setting time is the period between mixing the cement with water and the time when the mix has lost flexibility and has stiffened to some extent. In other words, it generally denotes the end of the period during which people can pour the wet mix into shape. The final setting time is the point at which the set cement has gained enough stiffness to withstand a certain pressure. Most standards provide for an initial minimum setting time of around 45 minutes at normal temperatures and a final setting time of no more than 10 to 12 hours.
Strength

The tests that determine the pace at which a cement acquires strength are often performed on a mortar that typically constitutes one part cement to three parts sand, by weight, combined with a specified amount of water. Tensile tests on briquettes, formed like a figure eight and thickened in the centre, complement compressive tests on cubical specimens or transverse tests on prisms.
The American Society for Testing and Materials (ASTM) requires a tensile test on a 1:3 cement-sand mortar and compressive testing on a 1:2.75 mortar. The British Standards Institution (BSI) recommends a compressive test on a 1:3 mortar or a concrete specimen as an alternative. On the other hand, the International Organization for Standardization (ISO) recommends a transverse test on a 1:3 cement-sand mortar prism, followed by a compressive test on the two halves of the prism that remain after it has been fractured in bending. Several countries use this cement testing strategy. All of these tests specify the sand’s size grading and, in most cases, its source.
Most tests for the minimum strength of cement are done at 3, 7, and sometimes 28 days. However, for rapid-hardening portland cement, a test at 1 day is sometimes necessary. Tests are necessary at 1 and 3 days for high-alumina cement.
Final Thoughts
Cement finds use as a binder in concrete, which is a fundamental material for all forms of building, including dwellings, roads, schools, hospitals, dams, and ports, as well as for ornamental purposes. These uses make cement an extremely essential man-made substance. We hope that this article has helped you understand the applications of cement, its manufacturing process, and the various types and tests used to determine its quality.
FAQs
What is the distinction between cement and concrete?
Although the phrases cement and concrete are sometimes used interchangeably, cement is actually a component of concrete. Concrete is essentially a combination of particles and paste. The aggregates include sand and crushed stone or gravel, while the paste used is a mixture of water and Portland cement.
What is the most prevalent type of cement?
The most popular type of cement is Ordinary Portland cement.
What are the uses of Ordinary Portland cement?
Ordinary Portland cement is the most commonly used type of cement, and it is appropriate for all conventional concrete construction. For example, people use it in pavements and sidewalks, bridges, railway structures, reinforced concrete buildings, tanks, reservoirs, sewers, water pipes and masonry units.
What exactly does “setting time” imply?
On combining cement with water, the resulting paste is fluid, or plastic, for a brief time. This paste substance may be reformed or remoulded during this period. The mixture stiffens and eventually hardens as the chemical interaction between water and cement proceeds. Setting time refers to this early stage of hardening.
What are the environmental impacts of cement manufacturing?
Cement manufacturing has a severe negative influence on the environment. These include emissions of airborne pollutants in the form of dust and gases, noise and vibration when operating machinery and blasting in quarries.
Cement manufacturing emits greenhouse gases directly through the generation of carbon dioxide when calcium carbonate is heated and indirectly through the use of energy, especially if the energy is derived from fossil fuels. The cement industry accounts for roughly 5% of worldwide man-made CO2 emissions, with the chemical process accounting for 50% and fuel combustion accounting for 40%. All in all, the cement industry emits about 900kg of CO2 for every 1000kg of cement produced.
Nitrogen oxide (NO2) released during the manufacturing process can cause or contribute to a wide range of health issues and negative environmental effects. This includes ground-level ozone formation, acid rain, water quality deterioration, global warming, and vision impairment. Children and people with lung illnesses such as asthma are among those who are affected, and exposure to these circumstances can induce lung tissue damage in those who work or exercise outside.
High quantities of sulphur dioxide (SO2) from the cement planets can impair breathing and worsen pre-existing respiratory as well as cardiovascular problems. Asthmatics, those with bronchitis or emphysema, youngsters, and the elderly are among the most vulnerable groups. SO2 also causes acid rain.
Lastly, carbon monoxide (CO) released during cement manufacturing, may be hazardous to one’s health by limiting the oxygen supply to the body’s organs and tissues. Furthermore, CO negatively impacts the cardiovascular and central nervous systems. It also contributes to the formation of smog, which can cause respiratory problems.
Several cement manufacturing businesses utilise equipment to decrease dust emissions during quarrying and cement production. Exhaust gas trapping, and separation equipment, are also becoming more popular.
Why is sand used with cement to produce concrete?
Sand of good quality adds to the density of mortars and concrete, thus preventing excessive mortar shrinkage. Furthermore. because it is an inert substance, it makes the structure more resistant to atmospheric agents.
Is cement harmful to humans?
Cement is very hazardous and can cause eye, skin, as well as respiratory system irritation. In addition, the calcium oxide in cement is corrosive to human tissue. Chromium, another ingredient in cement can induce serious allergic reactions.
How should cement be stored?
Proper cement storage allows for simple examination and identification. To keep moisture at bay, people store cement in weather-tight buildings. It should not be placed higher than 10 bags in a stack and should be organised in a header and stretcher shape. In addition, people must follow the “First in, First out” guideline while removing the bags to use.
What are the benefits of blending cement?
In water, the pozzolana material mixes with the lime and alkalies included in cement to generate a combination that contributes to its strength. It also makes the concrete less impermeable and resistant to sulphates. Furthermore, it improves workability, minimises bleeding, and limits the damaging expansion caused by the alkali-aggregate reaction. Free lime leaching also decreases. It decreases the heat of hydration, which helps to manage temperature differentials and inhibits the occurrence of cracks.
Who invented cement?
Although mankind knew about and used cementitious materials for centuries, it was a French inventor, Louis Vicat, who created artificial cement in 1817. He developed a precise procedure for combining and calcining the necessary elements.
What are the properties of good cement?
It is usually preferable to utilise the best cement in construction. Although acceptable cement attributes vary based on the kind of building, a good cement should have the following characteristics:
- Strengthens the stonework.
- Early stiffening or hardening.
- Possesses high plasticity.
- A fantastic construction material.
- Simple to implement.
- Excellent moisture resistance.
What are some cement shorthand?
| Shorthand | Chemical Formula | Common name |
| C3S | 3CaO·SiO2 | tricalcium silicate or alite |
| C2S | 2CaO·SiO2 | dicalcium silicate or belite |
| C3A | 3CaO·Al2O3 | tricalcium aluminate |
| C4AF | 4CaO·Al2O3·Fe2O3 | calcium alumino ferrite |
Share with your friends